Process mapping and management simplified
Identify, optimize, and drive efficiency across your organization with Process Manager.
Ease
Transform complex process maps, Visio charts, and procedure documents into clean, simple, and accessible process maps.
See how it's helped Coke FloridaCompliance
Alignment with the industry standard BPMN 2.0.
Read about Process ModelingOwnership
Embed process ownership, collaboration with feedback tools, and accountability, from the home, office or on the road.
Read about Process Manager MobileLooking to simplify process mapping and management?
Establish total process visibility, unearth risks and identify improvement opportunities, so you can gain control over all your business processes with Process Manager.

Process management simplified
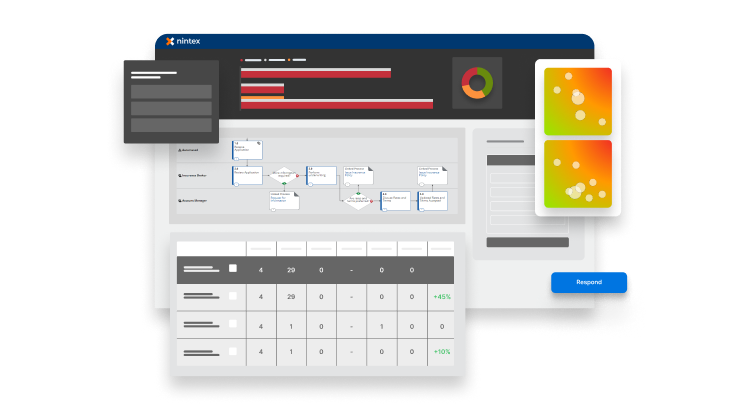
Process Manager delivers a ‘single source of process truth’ for all teams. The choice is yours: create simple, easy-to-understand process maps with the easy-to-use interface, or model more complex processes with BPMN.
Increase process ownership, collaboration, and accountability
Embed process ownership, collaboration, and accountability by handing process back to the business. Intuitive feedback tools will have employees engaging to optimize processes – all possible from the office, home, or on the road with the help of Process Manager Mobile App.
Reduce risk and ease auditing
Organization control and audibility will be improved with access to BPMN 2.0 industry standard modeling, mandatory approvals, escalations, and notifications. Safeguard operational impacts from staff turnover and knowledge loss and improve new employee onboarding.
Increase operational speed and efficiency
Map, evaluate, identify, and manage opportunities to improve your processes that will increase your operational speed, efficiency, and deliver continuous improvement.
Improve productivity with process maps
Personalized dashboards create total visibility of processes with live state changes. Get a real-time process health summary with team engagement stats and automatic tracking of every existing process, no matter their current state.
Latest process mapping resources
Accelerate your Process Manager onboarding
Our team of Process Manager experts will guide you in building a process excellence culture. Standard and Enterprise starter packs are available so you can tailor your onboarding approach to your specific business needs.
Rapid value implementation packProcess Manager health check
Bridging the gap between people, process and technology. If you are seeking to develop (or refresh) a clear and structured process management picture and need some help determining the real process health of your business, we’d love to help.
Read now